Topic 2: Crystal Structures
Part A: Lattice and basis in common crystals
Topic 2.1, 2.2
Structures: NaCl, GaAs, CsCl, BN: zip
A.1 Basis: Load your structure into Vesta. Wave your hand if Vesta is giving you trouble. Shrink the boundary to 0 - 0.99 in each direction. What is the Bravais lattice? How many basis atoms are in the cell? How many atoms are in the conventional unit cell? Sketch the cell.
A.2 Interpenetrating lattices: Open up the bonds menu, select the bond and delete it. Back to the main screen, on the upper left, select the "Objects" tab and turn off the cations. What is the anion sub-lattice structure? Repeat for the opposite atoms. (for BN, just consider this in-plane)
A.3 Asymmetric basis: Open up the Edit Structure (ctrl-E) and click on the 'Structure parameters' tab. How many unique atoms are specified? Mark them on your sketch. If you have an answer that is different than you got for A.1, what could explain this? Hint: Under the 'Unit cell' tab, the unit cell and space group are listed.
A.4 Symmetry removal: Go to 'Edit Structure-Unit cell" and set the crystal system to triclinic and the space group to P1. What, if anything, is different? What symmetry elements are now missing?
Part B: Hands-on crystal structures
Topic 2.1, 2.2
B.1 Bravais lattice: Identify the Bravais lattice for the crystal in your group.
B.2 Basis: What are the basis atoms for the crystal in your group? Define vectors for the lattice and basis and write a summation that will generate all atoms in a nanoparticle of this material with bounds of 10 unit cells on a side.
B.3 Distortions: If all the lattice parameters are the same length, what would happen to the Bravais lattice if you applied a force and stretched one of the lattice vectors? If your crystal has no equal lattice parameters, what would happen if you made them all equal?
B.4 Swap crystals: Trade crystals with a neighboring group; repeat questions above.
Part C: Slices
Topic 2.3
C.1 Initialization: Going back to the crystal structures of 2.A (above), load the GaAs structureinto Vesta. Wave your hand if Vesta is giving you trouble.
C.2 Sketch 1: Draw the structure in slices.
C.3 Origin shift: Draw the structure after shifting the origin to the other atom.
C.4 Coordination polyhedra: What are the coordination polyhedra created by the nearest neighbors for each site? Are the polyhedra point, edge, or face sharing?
C.4 Rocksalt lattice: Sketch NaCl using slices. What are the coordination polyhedra and are they point, edge or face sharing?
Part D: Miller Indices
Topic 2.4
D.1 Cubic system Draw (100), (111) and (102) planes for a cubic system. Check with your neighbors that you agree.
D.2 Hexagonal system: Draw (100), (111) and (102) planes for a hexagonal system. Check with your neighbors that you agree.
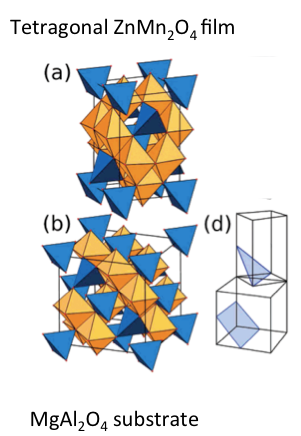
D.3 Epitaxy example: During my PhD, I grew some ZnMn2O4 single crystal films on MgAl2O4 single crystal substrates. With some work, I figured out the orientation between the films, as shown below. You can see the cations coordinated by 4 oxygen in a tetrahedra are shown in blue, octahedral sites are shown in orange. By inspection, you may be able to the blue tetrahedral sites of the ZnMn2O4 could replace the blue tetrahedral sites of the MgAl2O4. Per the drawing below, what two planes of the two structures are parallel?
The c axes are the same length and the base diagonal of the tetragonal cell is equal in length to the cubic cell.